Remember, the job market is competitive, so perseverance and continuous improvement are key. Good luck with your senior-level MES engineer job search!
Read MoreMonth: August 2023
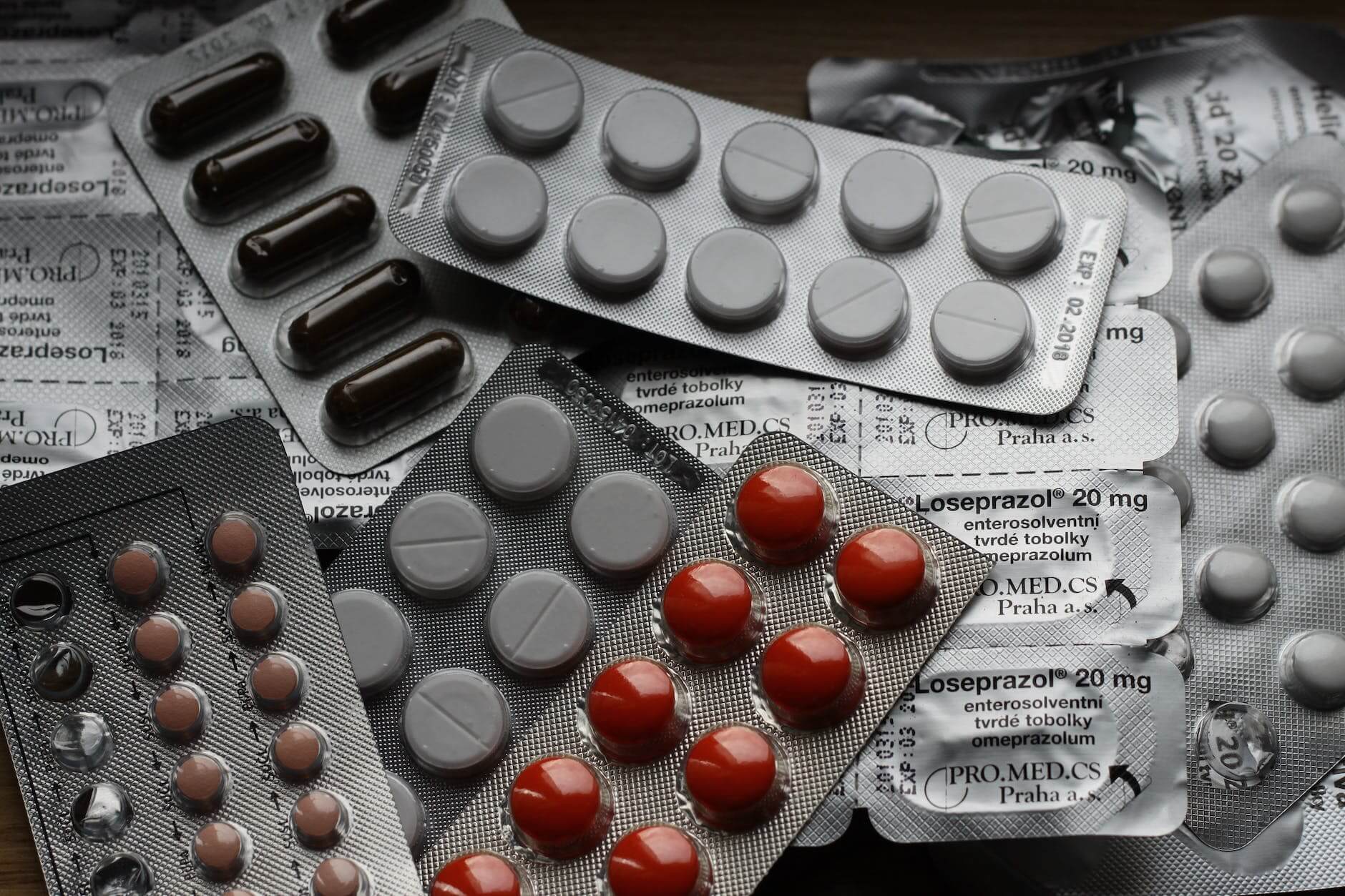
Understanding the Significance of 21 CFR Part 11 in GMP Compliance
Introduction In the highly regulated world of pharmaceuticals, biotechnology, and medical device manufacturing, ensuring product quality, safety, and data integrity is of utmost importance. One crucial regulation that governs electronic records and signatures is 21 CFR Part 11. This article aims to shed light on the significance of 21 CFR Part 11 in Good Manufacturing Practices (GMP) compliance. What is 21 CFR Part 11? 21 CFR Part 11, also known as Part 11, was established by the U.S. Food and Drug Administration (FDA) in 1997. It outlines the requirements for…
Read MoreMES 4.0 Explained
MES 4.0: Revolutionizing Manufacturing Operations In the era of Industry 4.0, Manufacturing Execution Systems (MES) have taken on a new form: MES 4.0. This technology-driven solution has the potential to revolutionize manufacturing operations, bringing unprecedented levels of efficiency, visibility, and flexibility to the shop floor. What is MES 4.0? MES 4.0 refers to the integration of traditional MES with the advancements of Industry 4.0 technologies such as artificial intelligence (AI), Internet of Things (IoT), cloud computing, and big data analytics. It extends beyond the traditional boundaries of the shop floor,…
Read MoreWhat does Emerson’s RTMS software do?
Emerson’s RTMS software stands for Real-Time Monitoring and Surveillance software. It is specifically designed to track and analyze data in real-time for the pharmaceutical industry. This software offers several benefits for the pharma sector: In summary, Emerson’s RTMS software positively impacts the pharmaceutical industry by enhancing safety, improving efficiency, ensuring regulatory compliance, and facilitating data-driven decision-making.
Read MoreWhat is LIMS and what does it do for the pharmaceutical industry?
LIMS, which stands for Laboratory Information Management System, plays a crucial role in the pharmaceutical industry by enabling interconnectivity across various laboratory processes. Let’s delve into more detailed information about the interconnectivity aspect of LIMS: In summary, LIMS in the pharmaceutical industry not only serves as a centralized data management system but also connects laboratory instruments, systems, and stakeholders. This interconnectivity enhances efficiency, data integrity, regulatory compliance, and collaboration, ultimately contributing to improved productivity and quality in pharmaceutical research, development, and manufacturing.
Read MoreJoin our Free Discord Chat for MES Professionals!
Are you a Manufacturing Execution System (MES) professional? Are you passionate about automation and improving production processes? We have an exciting announcement for you! Introducing our free Discord chat specifically tailored for MES professionals like yourself. Join us and connect with like-minded individuals who share your enthusiasm and expertise in manufacturing execution systems and automation. Why Discord? Discord provides a user-friendly platform where professionals can collaborate, share insights, and discuss the latest trends in MES and automation. With dedicated channels for various topics, you can easily engage in meaningful conversations,…
Read MoreMES Engineer Forums – Now Supports Tapatalk
Tapatalk is a popular mobile app that allows users to access and participate in various online forums, including the ones on MESEngineer.com. With Tapatalk, you can conveniently browse, read, and contribute to discussions in an organized and user-friendly interface. To download Tapatalk on Android, follow these steps: To download Tapatalk on iOS (iPhone or iPad), here’s what you need to do: Please note that app availability may vary based on regional restrictions. Enjoy your enhanced forum experience with Tapatalk!
Read More